What Is Psoriasis?
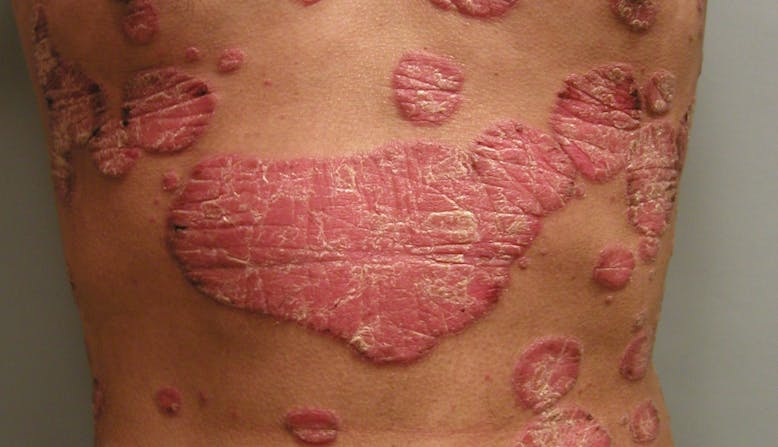
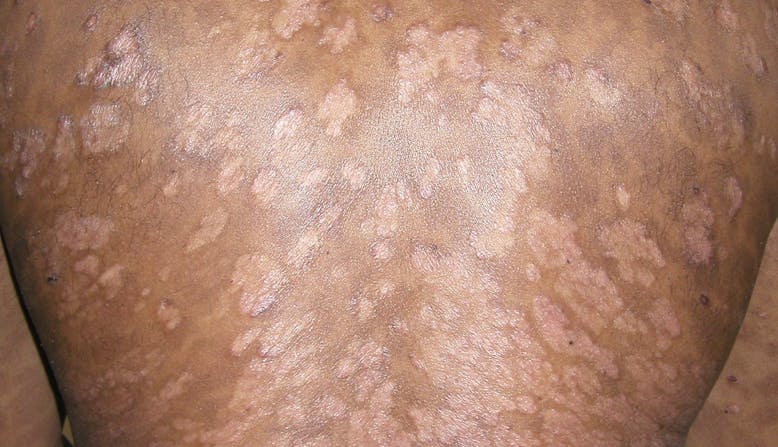
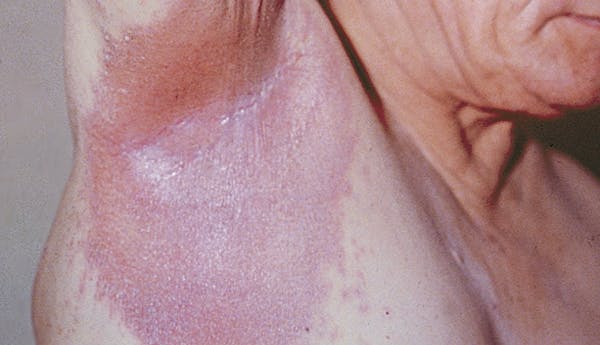
Psoriasis is an immune-mediated disease (a disease with an unclear cause that is characterized by inflammation caused by dysfunction of the immune system) that causes inflammation in the body. [1] There may be visible signs of inflammation such as raised plaques (plaques may look different for different skin types) and scales on the skin.
This occurs because the overactive immune system speeds up skin cell growth. Normal skin cells completely grow and shed (fall off) in a month. With psoriasis, skin cells do this in only three or four days. Instead of shedding, the skin cells pile up on the surface of the skin. Some people report that psoriasis plaques itch, burn, and sting. Plaques and scales may appear on any part of the body, although they are commonly found on the elbows, knees, and scalp.
Inflammation caused by psoriasis can impact other organs and tissues in the body. People with psoriasis may also experience other health conditions. One in three people with psoriasis may also develop psoriatic arthritis. Signs of PsA include swelling, stiffness, and pain in the joints and areas surrounding the joints. PsA often goes undiagnosed, particularly in its milder forms. However, it’s important to treat PsA early on to help avoid permanent joint damage.
Symptoms often start between ages 15 and 25 but can start at any age. Men, women, and children of all skin colors can get psoriasis.
Psoriasis Types
There are five types of psoriasis.
It is possible to have more than one type of psoriasis at one time and more than one type in a lifetime. Treatments may vary depending on the type and location of the psoriasis.
.png?ixlib=gatsbyFP&auto=compress%2Cformat&fit=max&rect=12%2C0%2C1175%2C675&w=778&h=447)
.png?ixlib=gatsbyFP&auto=compress%2Cformat&fit=max&rect=12%2C0%2C1175%2C675&w=778&h=447)
%20(7).png?ixlib=gatsbyFP&auto=compress%2Cformat&fit=max&rect=0%2C0%2C778%2C447&w=778&h=447)