Diagnosis
A health care provider will take several factors into consideration when making a diagnosis for psoriasis including:
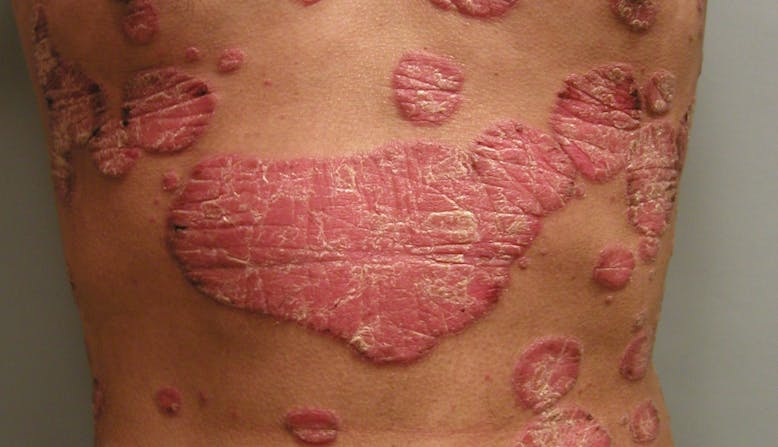
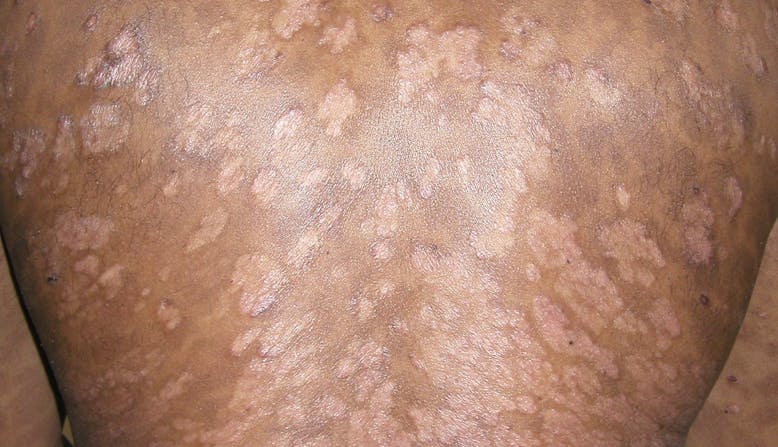
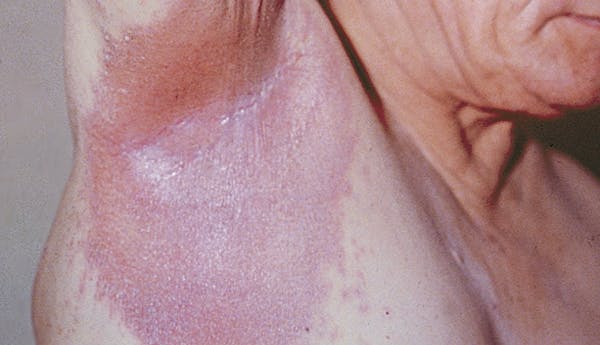
- The appearance of the skin. Psoriasis lesions (patches) may be thick, raised plaques, or fine scaling.
- The location of psoriasis. There are areas on the body that are more common for psoriasis to appear.
- Itch of the skin. This is a common symptom of psoriasis.
- A skin biopsy (the removal of a small piece of skin to be looked at under a microscope). The biopsy may also be done to determine if it is psoriasis or another skin condition.
Treatment & Management
Although there is no cure, there are more effective psoriasis treatments today than ever before. Treating psoriasis can help improve symptoms as well as lower the risk of developing psoriasis comorbidities such as psoriatic arthritis, heart disease, obesity, diabetes, and depression.
Treatments for psoriasis include:
Complementary and Integrative Medicine
Complementary and integrative medicine (CIM), which includes natural products, lifestyle changes, and mind and body practices is a popular addition to some people’s treatment plans. Talk with your health care provider before making any changes to your treatment plan to decide if it is right for you.
Prevention
There is no known way to prevent the onset of psoriasis, however, there are many ways that you can manage your triggers to reduce flares.
Outlook/Prognosis
Psoriatic disease is lifelong, and symptoms may resolve and recur throughout the lifetime.
While there is no cure for psoriasis, treatments today are more effective than ever before and research into new treatments, as well as a cure, is ongoing. Treating psoriasis can help improve symptoms and may decrease the associated inflammation that can lead to psoriasis comorbidities such as psoriatic arthritis, heart disease, and depression.



.png?ixlib=gatsbyFP&auto=compress%2Cformat&fit=max&rect=12%2C0%2C1175%2C675&w=778&h=447)
.png?ixlib=gatsbyFP&auto=compress%2Cformat&fit=max&rect=12%2C0%2C1175%2C675&w=778&h=447)
%20(7).png?ixlib=gatsbyFP&auto=compress%2Cformat&fit=max&rect=0%2C0%2C778%2C447&w=778&h=447)