The mission of the National Psoriasis Foundation is to drive efforts to cure psoriatic disease and improve the lives of those affected.
Over 8 million people in the U.S. have psoriasis. Nearly a third of them will develop psoriatic arthritis. A world without psoriatic disease and the related burdens is possible. We won't stop until you have the cure you deserve.





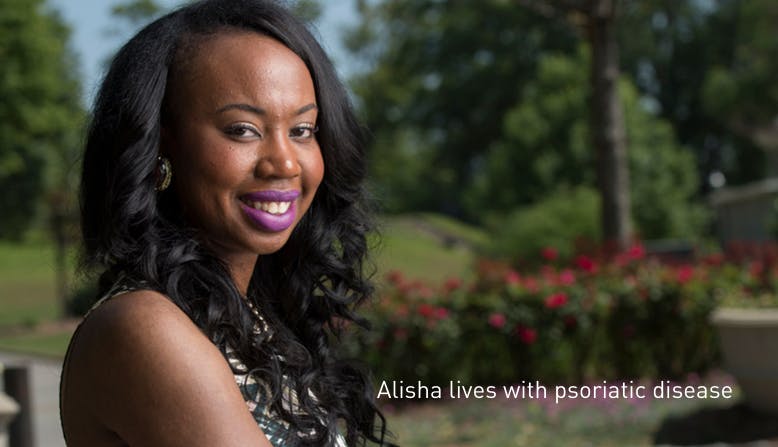
.png?ixlib=gatsbyFP&auto=compress%2Cformat&fit=max&rect=0%2C0%2C778%2C447&w=778&h=447)
%20(7).png?ixlib=gatsbyFP&auto=compress%2Cformat&fit=max&rect=0%2C0%2C778%2C447&w=778&h=447)